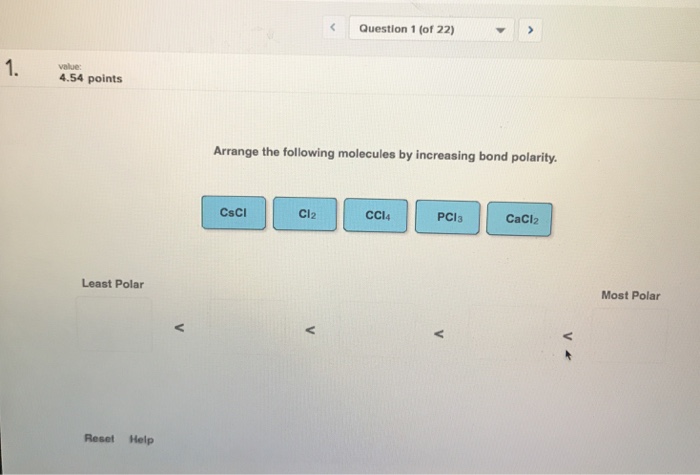
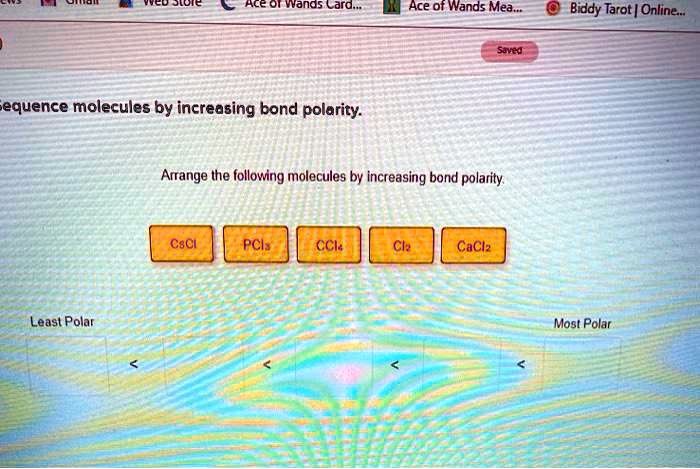
Arrange the following molecules by increasing bond polarity. – Arrange the following molecules by increasing bond polarity: a captivating journey into the realm of chemical bonding. Bond polarity, a fundamental concept in chemistry, plays a pivotal role in shaping molecular properties and influencing chemical reactivity. This exploration delves into the factors governing bond polarity, providing a comprehensive understanding of the underlying principles and their implications.
The concept of electronegativity, the driving force behind bond polarity, is thoroughly examined. Real-world examples of polar and nonpolar bonds illustrate the contrasting nature of these interactions. Factors such as electronegativity difference, bond length, and hybridization are meticulously analyzed, revealing their profound impact on bond polarity.
Bond Polarity
Bond polarity refers to the uneven distribution of electrons in a covalent bond. It arises due to differences in electronegativity between the bonded atoms, which is the ability of an atom to attract electrons towards itself. The more electronegative an atom, the greater its ability to attract electrons.
A polar bond is one in which the electrons are not shared equally between the atoms, resulting in a partial positive charge on one atom and a partial negative charge on the other. A nonpolar bond, on the other hand, is one in which the electrons are shared equally, resulting in no partial charges.
Factors Affecting Bond Polarity
The polarity of a bond is primarily determined by the electronegativity difference between the bonded atoms. The greater the electronegativity difference, the more polar the bond. Other factors that can influence bond polarity include bond length and hybridization.
Arrange Molecules by Increasing Bond Polarity, Arrange the following molecules by increasing bond polarity.
- H-Cl
- C-Cl
- N-Cl
- O-Cl
The bond polarity increases from H-Cl to O-Cl because the electronegativity difference between the atoms increases in that order. Chlorine is more electronegative than hydrogen, carbon, and nitrogen, so the bond polarity increases as the electronegativity of the atom bonded to chlorine increases.
Implications of Bond Polarity
Bond polarity has significant consequences on molecular properties. Polar molecules have a dipole moment, which is a measure of the polarity of the molecule. The greater the bond polarity, the greater the dipole moment. Dipole moments can affect intermolecular forces, such as dipole-dipole interactions and hydrogen bonding.
Polar molecules can also exhibit different chemical reactivity compared to nonpolar molecules.
Question Bank: Arrange The Following Molecules By Increasing Bond Polarity.
What is bond polarity?
Bond polarity refers to the unequal distribution of electrons in a covalent bond, resulting in a partial positive charge on one atom and a partial negative charge on the other.
What factors influence bond polarity?
The electronegativity difference between the bonded atoms, bond length, and hybridization are key factors that determine bond polarity.
Why is bond polarity important?
Bond polarity governs molecular properties such as dipole moments, intermolecular forces, and chemical reactivity, providing crucial insights into molecular behavior.